Disease Modifying Treatments
Prior to May 2018, there were no treatments available for spinal muscular atrophy (SMA), and type one was almost universally fatal before two years of age. However, over the last five years there have been very exciting developments in treatment which has resulted in a paradigm shift in the care and expected outcomes for patients diagnosed with SMA in 2021.
In Australia we have been lucky enough to be at the forefront of these new developments. As a consequence we have built up a wealth of experience and expertise across the country in the use of these new therapies and the ongoing management of SMA after treatment. As of September 2021, there are three disease modifying treatments which have been approved for use in Australia by the national regulators, the Therapeutic Goods Administration (TGA). At the time of writing, only two of these therapies have been listed on the pharmaceutical benefits scheme (PBS) and so, due to the extremely high cost of these drugs, in reality only two are really available to patients with SMA in Australia. Unfortunately, due to the relative lack of evidence for their use in the adult population, the regulatory bodies and funding committees have deemed that only patients commenced on these therapies whilst children are eligible to receive them through the PBS.
The three different therapies manufactured by three different pharmaceutical companies represent three separate approaches to treating spinal muscular atrophy. However, all three share commonalities: they all act at a genetic level and are specific to the commonest form of spinal muscular atrophy due to a change in the survival motor neurone gene, known as 5q SMA.
Genetics of 5q SMA
Spinal muscular atrophy is caused by a deficiency in a protein called survival motor neurone protein (SMN protein). This is produced from a gene on chromosome 5 called the Survival Motor Neurone gene. In humans, we have two variants of this gene: SMN1 and SMN2 genes. These genes are very similar to one another and have very minor differences. In nature, these differences mean that 95% of the protein produced by the SMN2 gene is a shortened and non-functioning version of the survival motor neurone protein. However, 5% of the protein it produces is a fully working version of the protein. This has led scientists to wonder whether we can increase the amount of survival motor neurone protein in the body by getting the SMN2 gene to produce more functioning protein.
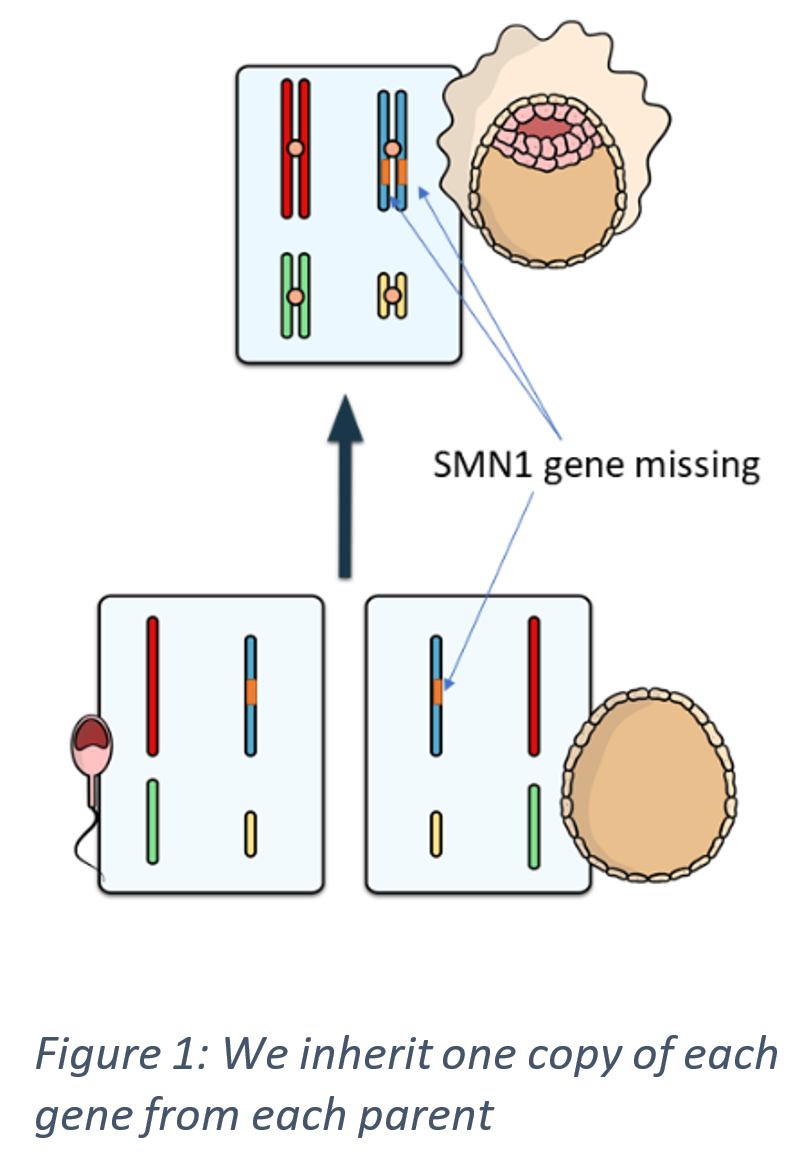
We have two copies of every gene in our body: one copy inherited from our father, and one copy inherited from our mother. In most cases of spinal muscular atrophy, the patient is missing both of their copies of the SMN1 gene. There is a subsequent lack of survival motor neurone protein which in turn causes the damage and death of nerve cells, resulting in weak muscles as seen in the patients with SMA.
There are two major approaches being taken at present towards correcting the lack of survival motor neurone protein within the body of patients with spinal muscular atrophy. These approaches aim to replace the missing SMN1 gene (this is referred to as gene replacement therapy) and increase the production of SMN protein by the SMN2 gene (this is referred to as up-regulation of the SMN2 gene).
Different therapies:
- Zolgensma (Novartis) – gene replacement therapy.
Zolgensma is administered by a single once off intravenous injection. There are a few important safety criteria that patients need to fulfil and there are a few short-term side-effects that need to be carefully monitored for. At the time of writing in September 2021, Zolgensma is not currently listed on the PBS and so is effectively not currently available for patients with spinal muscular atrophy in Australia. This approach is discussed in more detail in the Gene Therapy section of the website, which can be found by clicking the link here.
- Nusinersen / SpinrazaTM (Biogen) – SMN2 gene up-regulation by antisense oligonucleotide (ASO)
Nusinersen is administered by four monthly injections, into the spinal fluid (intrathecal) administered by a lumbar puncture (spinal tap). Nusinersen is safe and well-tolerated with the few rare reported complications being related to the lumbar puncture procedure rather than the medication itself. In patients with more advanced disease and scoliosis (curvature of the spine) or those who have had spinal surgery, lumbar punctures and intrathecal administration of Nusinersen can be technically challenging and may require the procedure to be performed under the guidance of x-rays by specially trained doctors.
- Risdiplam / EvrysdiTM (Roche) – SMN2 gene up-regulation by small molecule
Risdiplam is an oral liquid which is taken every day. Risdiplam has been shown in clinical trials to be safe and effective in treating spinal muscle atrophy.
Conclusion
There are pros and cons to each therapy, but on balance each therapy seems to work as well as the others in the treatment of spinal muscular atrophy. The important aspect to treatment is not which therapy is used but how early and how quickly it is started after symptoms have developed. The specific differences and which may work best for each particular patient should be discussed with the patient’s treating neurologist.
It is also essential that treatment is an adjunct to standard of care (attending regular medical appointments, having regular physiotherapy, regular occupational therapy, good respiratory management, good orthopaedic management and any other ongoing management that is deemed essential for individual patient care by the treating physicians). What has become very clear through collection of real-world data is that disease modifying therapies do not work in isolation and require good quality allied health therapy and medical care to maximise the benefit.
For further information please discuss with your treating neurologist or neuromuscular specialist nurse.
